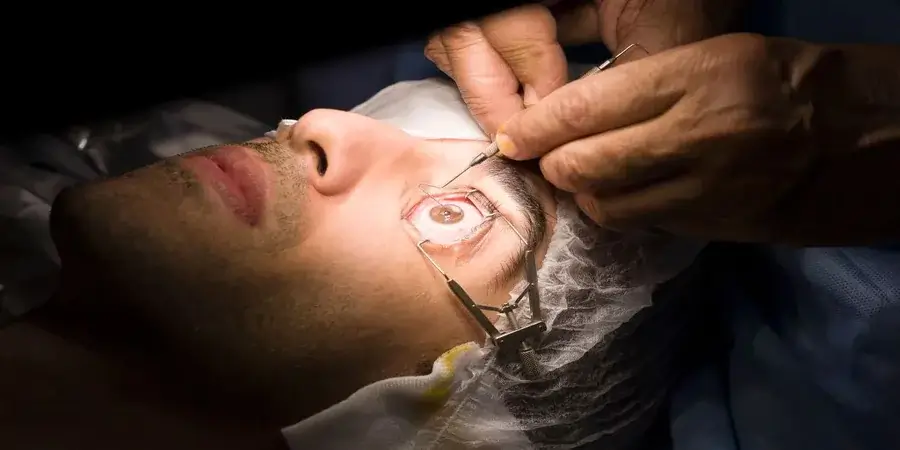
The development of a medical device is far more than a technical challenge — it is a complex, highly regulated process that requires careful planning, early testing, and performance validation. From the first conceptual model to the final clinical evaluation, each stage is essential for ensuring that the product not only works but does so safely and effectively.
One of the most important early steps in this journey is the medical device feasibility assessment, followed by comprehensive performance testing. These elements, along with operational execution from trusted regional partners, form the backbone of modern device innovation.
Why Does Feasibility Matter in Medical Device Development?
Before investing in large-scale clinical studies, developers must determine whether their device is viable — both technically and clinically. Feasibility studies are typically small, early-phase trials designed to answer practical questions about a device’s usability, safety, and integration into healthcare workflows.
These assessments are especially crucial for novel technologies or devices targeting complex user populations, such as elderly patients or individuals with chronic conditions. Without feasibility data, companies risk entering expensive trials with flawed assumptions.
Feasibility studies help address key concerns such as:
- Can the device be operated easily by clinicians or patients?
- Are there early indications of safety issues?
- Will the trial design work in a real-world clinical setting?
This data informs both device refinement and study design, helping developers make better decisions and reduce the risk of costly delays or trial failure.
How Is Performance Testing Structured and Evaluated?
Once feasibility is established, the next step is to validate how well the device performs its intended function. Medical device performance testing involves assessing the reliability, precision, and accuracy of the product under controlled conditions — ideally replicating the environment in which it will eventually be used.
This process may include both bench testing and clinical trials, depending on the device type and regulatory classification. For high-risk or implantable devices, clinical performance testing is mandatory and often forms the core of the regulatory submission.
Performance testing typically includes:
- Objective comparisons against a gold-standard device or method
- Quantification of sensitivity, specificity, and user error rates
- Analysis of signal consistency, device durability, or software reliability
- Observation of user feedback and performance variation across clinical settings
The resulting data must demonstrate not just functionality, but safety, reproducibility, and user benefit. These outcomes are critical for achieving regulatory approval and market access.
Why Poland Has Become a Strategic Hub for Device Trials
As medical device development becomes more globalized, many companies seek clinical partners who combine regulatory familiarity with operational efficiency. Engaging a CRO in Poland has become a popular strategy, thanks to the country’s strong clinical infrastructure, EU alignment, and experienced research professionals.
Poland offers a number of advantages for device sponsors:
- Access to highly trained investigators and research nurses
- Competitive trial costs with high data quality standards
- Rapid patient recruitment, especially in urban academic centers
- Centralized ethics and regulatory processes compatible with EU MDR
- High English proficiency among trial staff and site personnel
Local CROs are familiar with the specific requirements for both feasibility studies and performance trials, helping sponsors navigate submissions, recruit appropriate sites, and deliver validated data efficiently.
What to Look for in a Regional CRO
When selecting a partner for medical device trials, especially in Central Europe, consider the following:
- Experience with device-specific protocols and regulatory pathways
- Established relationships with hospitals and device-specialized investigators
- Access to data management and biostatistics teams familiar with MDR and FDA requirements
- A history of successful submissions and passed audits
- Flexibility in adapting to novel devices or decentralized elements
A capable CRO in Poland can reduce trial timelines, ensure compliance, and build local partnerships that support future market entry.
Combining Clinical Rigor with Market Strategy
Today’s medical device developers are not just solving engineering problems — they’re navigating a landscape shaped by strict regulations, tight timelines, and increasing demands for real-world evidence. By integrating early medical device feasibility research with rigorous medical device performance testing, and partnering with experienced regional CROs, sponsors can move through development more confidently.
This strategic alignment helps innovators get products to market faster, with stronger evidence and fewer regulatory setbacks — ultimately improving patient care and creating a competitive edge in the evolving medtech landscape.